The exact amount of time that it takes for a diamond to form is unknown. The truth is that every diamond takes a different amount of time to form

Diamond Formation & Creation
There’s a common myth out there which associates diamond formation with coal formation. It’s believed by many that diamonds are simply pieces of coal that have been transformed under a very high pressure and high temperature. This is far from the truth. One reason why is because coal is formed from the remains of early vegetation. Diamonds, on the other hand, are much older than the plants which are the main ingredient for the formation of coal.
So, if diamond and coal formation aren’t interconnected, how are diamonds formed? Most diamonds are found in commercial mines, and their deposits were formed inside the Earth’s mantle. Diamonds are brought to the surface of the Earth whenever an event occurs such as a violent volcanic eruption. They are brought to the Earth’s surface in large chunks of rocks that contain diamonds, and these are called xenoliths.
Before the eruptions, diamonds are created in something called a “diamond stability zone.” This zone is found in the upper mantle of the Earth. Diamonds need extremely high temperatures to form, which is why this zone has a temperature of over 1,000 degrees Celsius. It’s also extremely high pressure. This natural environment for diamond formation is found around 150 kilometers below the Earth’s crust, which is extremely deep.
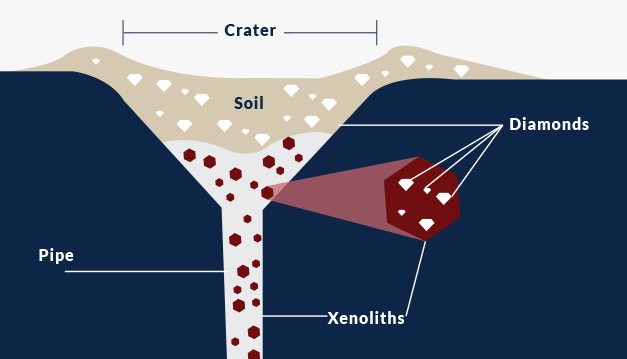
Where Else Do Diamonds Form?
Diamonds don’t just form in the Earth’s mantle - there are other scenarios where diamond formation is also possible. One of the most intriguing modes is the formation of diamonds in the outer reaches of the solar system. Researchers at NASA have found extremely small pieces of diamonds in extraterrestrial bodies. These have formed either in outer space or in the mantle of other planets.
Apart from the Earth’s mantle, there are a couple of other obscure places that provide the necessary qualities for diamond formation. For example, some diamonds have been found in subduction zones, where two different tectonic plates come together. All diamonds formed in the earth are quite old. Many diamond formations took place in the first couple billion years of the Earth’s history. Some diamond deposits are younger, being hundreds of millions of years old, compared to a couple billion years old.

A diamond itself can’t be dated to find out its exact age. However, you can look at the inclusions of other minerals in a diamond to determine its age. If the inclusions contain specific elements such as potassium, the element can then be used in a radioactive dating scheme. Almost all diamonds are at least hundreds of millions of years old, with many of them being between 1-3 billion years old.
Most diamonds were formed at a time when the Earth was much hotter than it is today, and when conditions were more favorable and appropriate for diamond growth and formation. Some diamonds take short periods of time to form, such as days, weeks, or months. Other diamonds take years to form, even millions of years.
This is because growth isn’t always a continuous process. Diamonds might start to grow, and then for whatever reason, an interruption might occur. Maybe the temperature changed, or the pressure. The diamond will then stop growing, and it could sit for hundreds, even millions of years before it starts growing again.
Diamonds are always growing and forming in the Earth’s mantle, just like they have been for billions of years. However, it could be millions of years before the diamonds that are forming today appear at the surface of the Earth. As the diamonds form deep in the earth, they withstand exposure to all sorts of gasses, minerals, and other materials surrounding the ore. Although the budding gem hardens to an unbreakable state, any little thing that touches it during this phase has an impact on the color of the gem. In fact, most diamonds appear white, but upon further inspection, some notice that the gem shines with a yellowish tint, or that the brilliance is a light, almost imperceptible shade of brown.
This does not mean that there is anything wrong with the diamonds; they are just unique in their beauty. This is just a perfect example of how mother nature produces things with flaws that add to their unique beauty and sparkle! Diamond Strength & Chemistry The chemical formula of diamond is C. This is the chemical symbol for the well-known element, carbon. However, graphite and soot are also made of pure carbon atoms.

So, how do you differentiate between soot, graphite, and diamond if they are all made up of the same carbon atoms?
Carbon atoms can be arranged in many different physical forms, which are called allotropes. These different arrangements are how graphite, soot, and diamond differ from each other so greatly, even though they are all made up of the same thing. In a diamond, the carbon atoms are all covalently bonded to one another. They produce a three-dimensional network solid. This strong allotrope differs from the allotropes of graphite and soot, which are more relatable to chicken wire, making them softer and not as strong.

Diamonds are the most concentrated form of pure carbon in the natural world. They are the strongest mineral known on earth. Even though soot and graphite are made from pure carbon as well, diamond is stronger due to its allotrope. All carbon atoms have four valence electrons that are available for bonding. In diamonds, each of these four electrons forms a covalent bond with a valence electron that belongs to a neighboring carbon atom. Since all the electronics are bonded so tightly and uniformly, it creates the hardest mineral on earth - diamond. To sum it up, the extremely tight formation and bonds of the carbon atoms in diamonds are the reason why they are so strong.